|
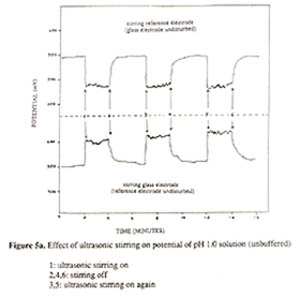
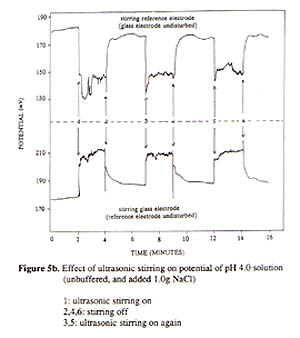
COUNTERION TRIPLE LAYER FOR EXPLAINING STIRRING AND TEMPERATURE EFFECTS ON pH MEASUREMENTS K. L. CHENG Department of Chemistry, University of Missouri-Kansas City, Kansas City, Missouri 64110 Abstract This paper reports the pH glass electrode potential based on the double capacitor theory and a new, novel concept of mobile countertion triple layer next to the double layer to explain the electrode potential changes caused by stirring and temperature. This mobile triple layer behaving like a rubber band may be removed by stirring or agitation and temperature and returned after stopping stirring or lowering temperature. Stirring and temperature affect differently the potential of the pH and SCE electrodes for their different mechanisms. Contrary to the past, results reported here were obtained from the experiments of glass electrode and SCE in two separate beakers connected with a conducting wire so that stirring one electrode while keeping the other unstirred. There is a weak charge attraction between the double and triple layers, not a strong covalent bonding for complexation so that the latter can be removed by stirring or temperature. In considering the present results and the new concept, the Boltzmann equation has been modified to include the net charge density and the triple layer potential. The counterion triple layer concept will have a tremendous impact on understanding the interface structure and properties and stimulate further studies and applications for ISE. Introduction Though the effects of stirring and temperature on the pH glass electrode potential have been known for a long time and discussed in literature (1), we have found conflicting recommendations on stirring in the pH measurements (4,5). Ineffective efforts for compensating the stirring and temperature effects have also been suggested because the past researchers did not understand their fundamental mechanisms. Although the double capacitor theory for the pH glass electrode potential has been proposed more than a decade ago, and ignored by most quantitative textbooks which still describe the redox and Nernst equation for the ion selective electrodes (ISE) (6,7) instead of the capacitor and the Boltzmann equation (6). Many electroanalytical chemists still believe the speculative redox reactions and mechanisms for the common reference electrodes, as a result, the stirring and temperature effects cannot be properly explained by the Nernst equation (7,8). This paper will demonstrate the important role of correct conception and mechanism for explanation of electrode phenomena. Hope this paper will stimulate electroanalytical chemists to be open-minded and encourage new challenges. Experimental A Corning pH/ion meter 150 was used for pH and potential measurements. A magnetic stirrer and Branson ultrasonic cleaner B-12, 50/60 Hz were used for agitation. In the study of stirring effect on pH glass electrode and SCE reference electrode, putting both electrodes in two separate beakers containing the same 50 mL solution. The two solutions were connected with an insulated conducting wire. For studying the stirring effect, one beaker was stirred while the other unstirred. Results and discussion Application of a pressure in an electrolyte solution gives rise to a potential difference and corresponding electric field. This is the phenomenon commonly called streaming potential (2,3) which has been applied to the stirring effect (2,3). F = P ( z / k(10 -6 ) ) (1) where P is the pressure in mm Hg and (F is the zeta potential in mV. The streaming potential has been applied in the literature with a capillary mode and may not fit into the current non-capillary mode. It seems that the streaming potential equation cannot be applied to the commonly stirring effect on glass and SCE electrodes. The double layer concept has been widely discussed, a new couonterion triple layer concept is suggested here as shown in Fig. 1. Except the adsorbed ions in the inner layer are not in the hydrated form, the ions in the double and triple layers are in the hydrated form (not shown in Fig. 1). This triple layer containing counterions is attracted to the double layer by the charge attraction force, not the covalent bonding complexation force. This is evidenced by its mobile characteristics. Its thickness is controlled by its counterion concentration, temperature, solvent, and other factors. Because of its characteristics, the present results suggest the presence of the counterion triple layer concept. It can explain the stirring and temperature effects on ISE and may be applied to other cases. The stirring effect results are shown in Figs. 2-6. As reported previously (6,7), as a capacitor, in an acidic solution the pH glass electrode is adsorbed with H+ and in a basic solution it is adsorbed with OH- showing a positive or negative potential, respectively (9). Its potential may be derived by the modified Boltzmann equation (10). In an unstirred solution, the electrode potential is E unstirred = E i + E d - E cit (2) where E i , E d and E cit are inner layer potential, double layer potential, and counterion triple layer potential, respectively. In a stirred solution, the electrode potential will be E stirred = E i + E d (3) Then Ecit = E stirred - E unstirred (4) Stirring actually enhances the electrode potential by removing the counterion triple layer effect. The difference between the stirred potential and the unstirred potential is the counterion triple layer potential which is illustrated in Figs. 2-5. In an acidic solution, the electrode is positively charged, at the interface, the counterion anions will compensate the electrode potential (or charge density) resulting in a less positive potential. In a basic solution, the electrode is negatively charged, at the interface, the counterion cations will compensate the electrode potential resulkting in a less negative potential, The stirrignand temperature effects as a function of pH are in a linear relationship as shown in Figs. 3- 4. When the solution pH went to more acid the stirred potential increased to more positive and pH went to more basic, the stirring potential moved to more negative. As shown in Fig. 5, the stirring effect is reasonably repeatable. At pH 10, the stirring effect showed somewhat unevenly. This might be due to somewhat different adsorption forms for H+ and OH- ions.
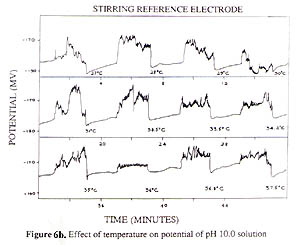
A special attention should be made that in comparing the stirring effects of glass and SCE electrodes, they had an opposite effect (Fig. 4). The intersects for the two curves in Figs. 2 and 4 is not at pH 7, instead, at approximately pH 6.5. This is in agreement with the isoelectric point of glass at around 6.5 ( 7 ). In the literature it has been mistakenly assumed that the isoelectric point of pH glass is 7 ( 4, 12, 13 ). One must realize that the stirring effect on the two electrodes is not the same, having different directions. So it is difficulty to study and compensate the stirring effect for various pH values while the two electrodes are in the same solution or using the combination electrode. It was possible for us to study the stirring and temperature effects by putting the two electrodes in two separate beakers. Results of the stirring and temperature effects on the glass electrode and SCE are shown in Fig. 3 indicating that stirring and temperature could remove the effect against the electrode potential providing that the reference electrode potential is kept constant. The stirring effect on the SCE and glass electrode at different temperatures are quite different (Figs. 6a and 6b). The ultrasonic stirring of the SCE at pH 10 gave very unstable potentials (6b) because the Hg 2Cl2 is relatively separated far away from the sample solution. The SCE is negatively charged due to adsorption of Cl-. The stirring would remove the counterion triple layer of cations, but also the part of KCl so that the SCE went to more positive. The junction potential at the tiny holes of the SCE might have had discontinuous changes of KCl concentration, non-equilibrium situation. The potential changed to more positive indicates the removal of chloride anions from the KCl solution at the junction. At higher temperatures the junction holes become larger making stirring easier to move away more chloride anions diluting the KCl concentration. So the resulting SCE potential became more positive and relatively stable. This also confirms that SCE behaves as a capacitor of Cl- ISE, not a redox electrode. It is desirable to develop a stable reference electrode resisting environmental changes.
Both Nernst and Boltzmann equations do not explain the stirring and temperature effect mechanisms, they only show the temperature slope. The modified Boltzmann equation includes the net charge density (10). Now, in consideration of the presence of counterion triple layer, it may be further modified, E unstirred = 2.3 kT/ (Z ?e) log (Ni/No) - E cit (5) where Z ?is the net charge density for the zwitterionic electrode surface and E cit is the counterion triple layer potential. One should remember that the Boltzmann equation only provides the electrode potential without considering the counterion triple layer effect which always exists in a practical, unstirred electrolytic solution. In other words, the potential calculated from the Boltzmann equation based on the electrode surface charge density is actually the potential of stirred solution without the counterion triple layer effect. The counterion triple effect differs from other triple layer models. For instance, Hayes (11) described his model as surface complexation model (SCMS) that are used to describe the partition of inorganic metal ions between mineral and aqueous phases. These models are based on the premise that minerals have surface functional groups capable of forming complexes with ions in the interface region. Our triple layer model is mobile counterion phase with an opposite charges to the double layer, not related to metal ions for stable mineral complex formation. Conclusion A counterion triple layer concept has been proposed for explaining the stirring and temperature effects on pH glass electrode potential measurements which have not been adequately explained. It may also be applied to other cases. The pH glass and the SCE reference electrodes are considered as capacitors, their potentials are related to the capacitance potentials, E = q/C. This counterion triple layer is the one containing counterions next to the double layer and may be removed by stirring and temperature. It is mobile like a rubber band, returning to the original potential after stopping stirring or lowering the temperature. The Boltzmann equation has been modified to include the net charge and counterion triple layer effects. Removal of the triple layer enhances the electrode potential. Experiments using both glass electrode and SCE in two separate beakers made it possible to obtain the results reported here, because the two electrodes have different effects on potentials at different pH values The new concept seems to explain well the stirring and temperature effects on the pH measurements. Its applications to other cases are anticipated. Any concept, theory, or mechanism must be able to explain phenomena correctly, otherwise, they should be modified or discarded. References [1] Ching-I Huang, Hsuan Jung Huang, K. L. Cheng, Chapter in T. F. Yen, et al (editors), Advances in the Applications of Membrane-Mimetic Chemistry. Plenum Press, New York, (1994), pp 227-240. [2]. J. O’M Bockris and A. K. N. Reddy, Modern Electrochemistry. Vol. 2, Plenum Press, New York,(1977). [3] D. R. Crow, Principles and Applications of Electrochemistry. Chapman and Hall, New York, (1988), 3rd edition. [4] "Orion Research Analytical Analysis Guide" , 4th ed. Cambridge, Ma. October (1972). [5] R. A. Durst, ed. Ion Selective Electrodes. National Bureau of Standards, Special Publ., 314, Washington, D. C. (1969). [6] K. L. Cheng, Microchem. J., 42, (1990) 5. [7] K. L. Cheng, pH glass electrode and its mechanism in Electrochemistry, Past and Present. J. T. Stock and M. V. Orna, (Eds.). ACS Symposium Series 390, pp. 286-(1989). The Second Nernst Hiatus, paper presented at the Pittsburgh Conference, 1992. [8] Khalid R. Temsamani, K. L. Cheng, Challenges to conventional redox mechanisms of calomel and Ag/AgCl reference electrodes. Paper presented at the Colloid and Surface Chem. Div. , 215th ACS national Meeting (1998). Dallas, TX. [9] K. L. Cheng, H. Z. Song, S. X. R Yang, J. Chem. Soc. Commun., (1988) 1333. [10] K. L. Cheng, Microchem. J., 59 (1998) 457. [11] K. F. Hayes, Equilibrium, spectroscopic, and kinetic studies of ion adsorption at the oxide/aqueous interface. Dissertation, Stanford University, Stanford, Ca. ( 1987). [12] "Practice and Theory of pH Measurement", Ingold, Ordorf, Switzerland (1989). [13] "Handbook of Electrode Technology", Orion Research, Cambridge, Ma. (1982). |